In oncology R&D, speed and adaptability are non-negotiable. While the B16F10 model is a critical tool for evaluating tumor progression and immunotherapeutic strategies, the completed data package is only as valuable as the efficacy model can show.
How We Deliver Personalized, Experimental Data at the Speed of Right®.
We are scientists, just like you. Structured differently from global CROs, we provide ease of execution, data delivery, and study insight to get you to the next step as efficiently as possible.
How do we do it?
Instead of trading personalized service for integrated data, we provide both by giving you:
- A Single Point of Contact: A dedicated scientist in our Boston lab will work with you, starting with study design, test article availability, model selection/development, PD, and PK/PD data generation as the final deliverables, providing ease of communication and peer-to-peer partnership.
- Efficacy & PK/PD Data, Fast: Our integrated facility and technology platforms—combining In Vivo (AAALAC-accredited), industrial standard LCMS, and ligand binding and molecular assays under one roof—ensure seamless data flow. Your complete PK/PD data packages are generated in a structured format specifically designed to streamline and accelerate your IND submission timeline.
- Agility and Flexibility: We don’t just talk about flexibility; we execute it. We routinely start studies in as little as 24 hours when the TA arrives at our facility, and our agility in deploying analytical platforms to obtain the essential data to answer the questions and maximize learning and minimize costly restarts, ensuring you get the most out of every cohort.
The Meadowhawk Approach to B16F10 Studies
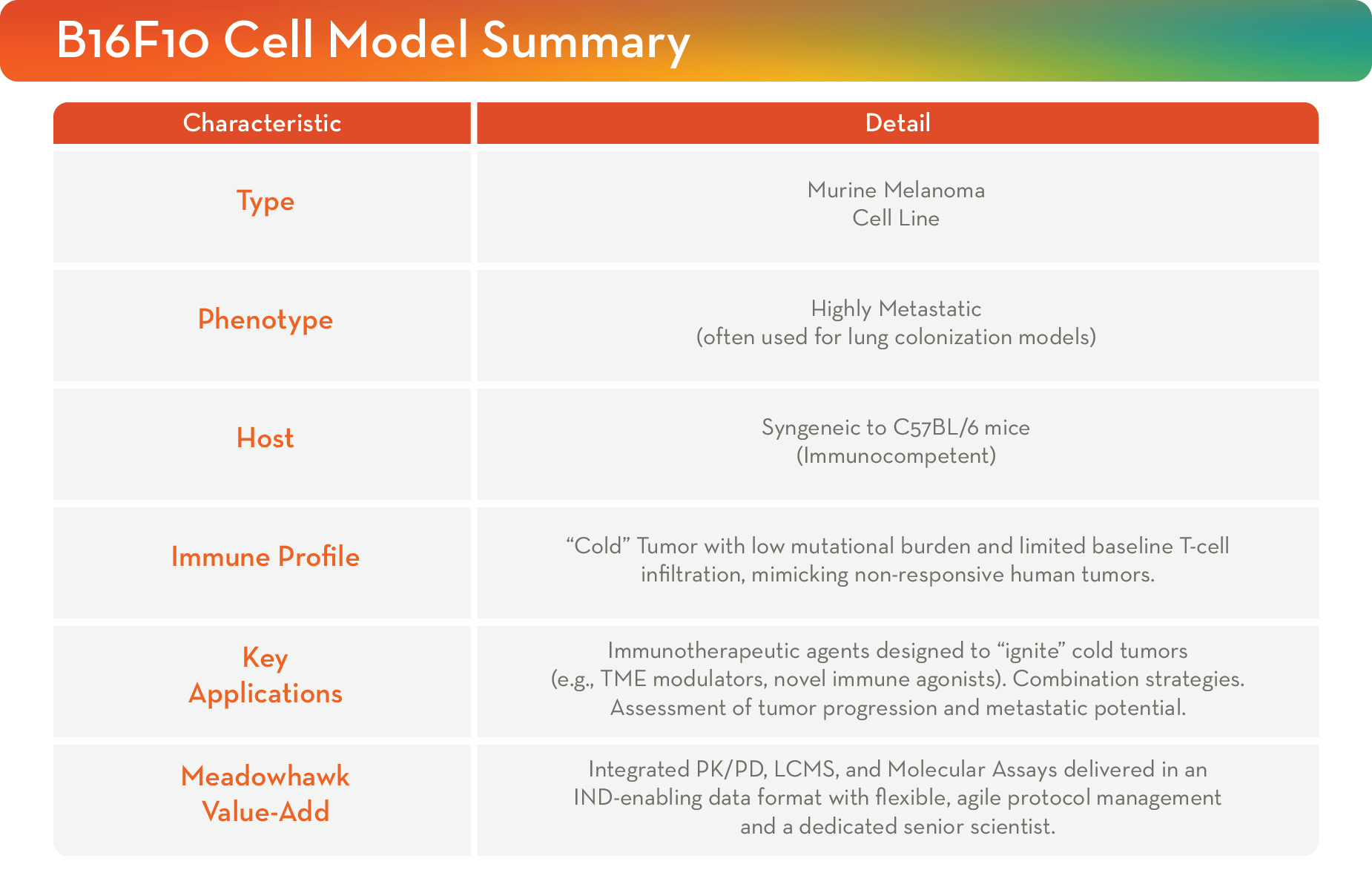
At Meadowhawk, the B16F10 syngeneic model is leveraged to provide clarity where it counts. We accelerate decision-making with actionable, reproducible data that demonstrate the preclinical efficacy and provide insight for clinical advancement.
Interrogating the Cold Tumor Microenvironment: The B16F10’s low mutational burden and limited baseline T-cell infiltration make it a challenging, yet highly relevant, model for testing novel agents designed to “ignite” unresponsive tumors. Our experts design studies to effectively:
- Drive next-gen immune modulator and immunotherapy development.
- Reveal dynamics of immune cell infiltration and tumor-stromal interactions.
- Test combination strategies with standard checkpoint inhibitors.