Overcoming Discovery Assay Challenges
Accurately assessing the biodistribution and pharmacokinetics of candidate therapeutic compounds during drug discovery can be very challenging but is essential for studying their stability, efficacy, and safety. Decision making at the early discovery stage resides heavily on fast and reliable study data/ results, ideally within days. For novel chemical entities, we initially design and implement “diagnostic probes” to ensure the data quality and fast delivery. Such an approach has been adopted into analyzing other modalities as well.

Decision making at the early discovery stage resides heavily on fast and reliable study data/ results, ideally within days.

Improving Assay Reliability & Turnaround Time
To overcome the perception that discovery assays can be crude, less reliable, and problematic in quick turnaround time (TAT) situations, these diagnostic probes are developed and deployed. They are integral to early screening assays as a default approach to enhance data quality and subsequently facilitate troubleshooting. These diagnostic probes include sample dilution, internal standard cocktail, formulation “QC”, stability QC, blood plasma partitioning (B-P), sample triple extraction and triple injections. These measures are designed to shed light on the data quality and address potential issues early in the assay development process.
Accelerating LC Method Development
Due to the unknown nature of testing compounds, the cocktail internal standard solution makes LC method development easier and quicker. Additionally, three types of diagnostic probes performed consistently in early stage sample analysis (as volume permitted).
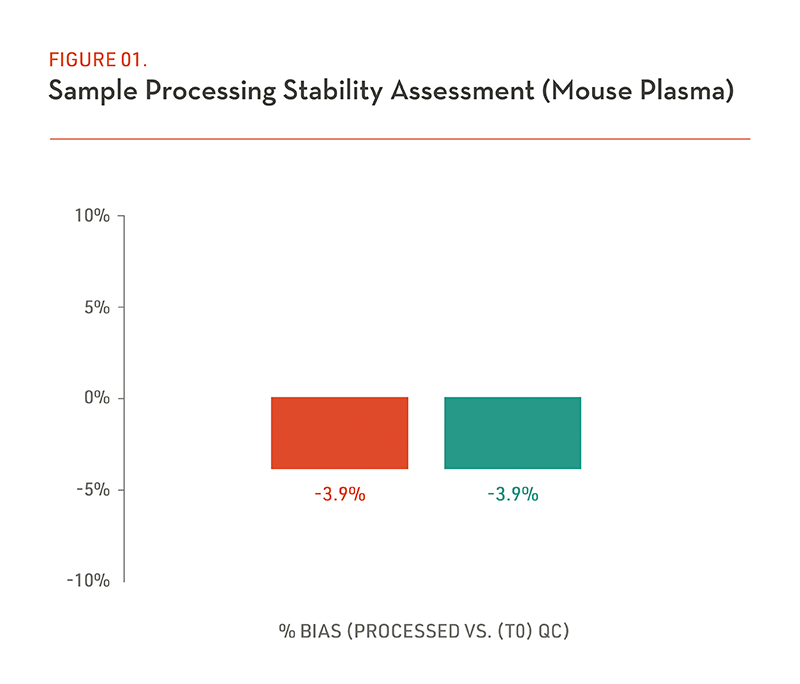
(Figure 1) Testing compound and sample short term stability using T(0) QC as a reference as result shown, that two different testing compounds do not exhibit stability issues in mouse plasma in comparison with T(0) QC sample during sample preparation process. In addition, blood plasma partitioning measurement can also be conducted at the client’s request when our in vivo team carry out the in-life part of PK study.
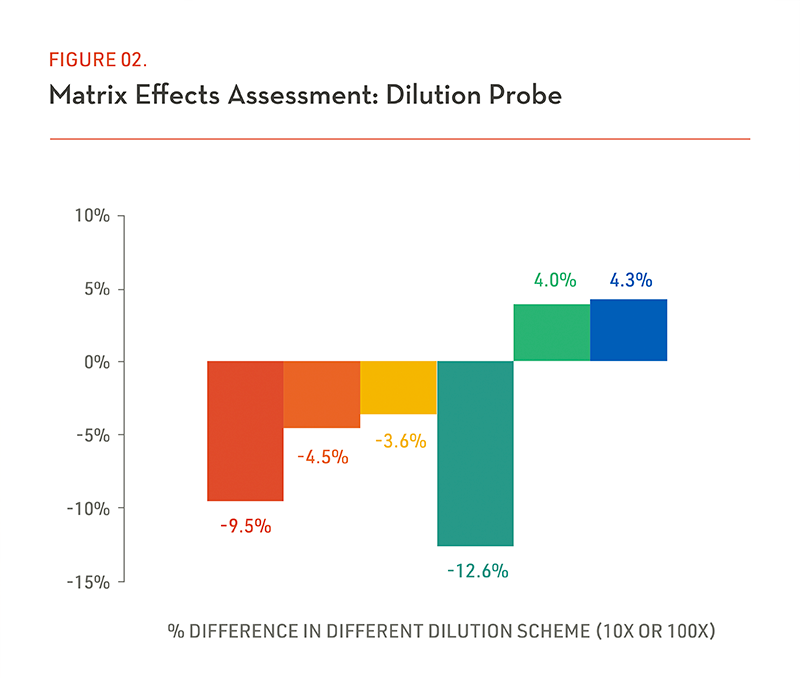
(Figure 2) In order to get assessment of well known matrix effects elected study samples (often in early time points) undergo dilution at 2 different levels (with sufficient sample volume).and assayed three times. The example graph shows the % CV and direct comparison with different dilution factors.
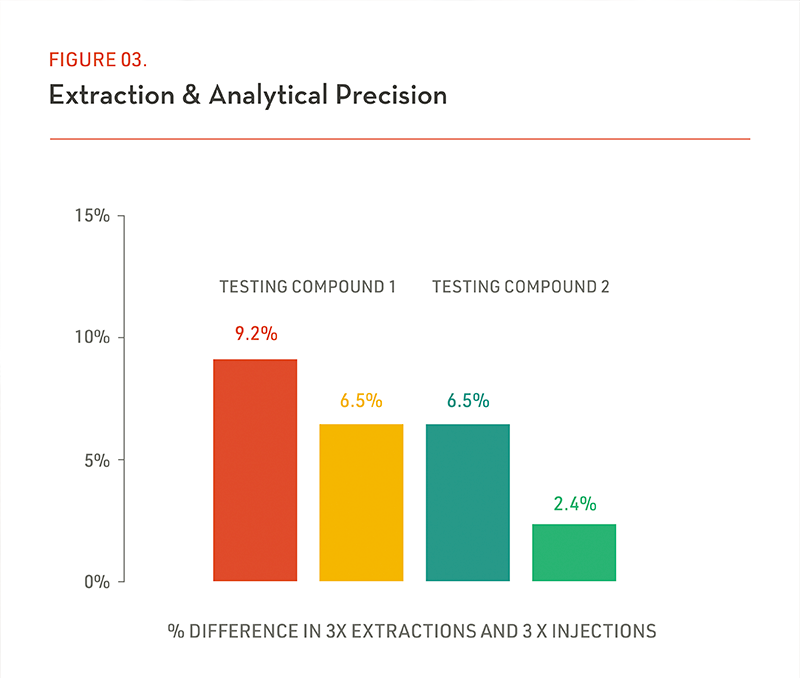
(Figure 3) To ensure the assay robustness, a single sample can be processed 3 times and injected three times from one single well to illustrate the reproducibility of the assay). This means 3x process of ONE single sample (with sufficient sample volume) and 3x injections with the same process sample (from one well). This graphic performance result sheds light on the sample process and instrument acquisition.
Get Expert Opinions & The Latest Trends
Creating personalized paths that get therapies to marketing on time and on budget means staying ahead of the curve. Get thought provoking articles, expert insights and the latest news from our senior scientists.
