Overcoming the Antibody Drug Discovery Bottleneck
Antibody (immunoglobulin)-based biologics are among the most commonly approved biologic drugs. However, their discovery and development process is slow due to various factors, including the complex and diverse structures and mechanisms of action within the body. During the discovery phase of these biologics, pharmacokinetic (PK) and pharmacodynamic (PK-PD) studies are crucial for optimizing delivery and target engagement to identify lead candidates. Quick access to high-quality, reliable PK data is essential for advancing rapidly through this initial stage.
Ligand binding immunoassays (LBAs) are the preferred method for measuring antibody levels in samples to gather PK data. Unfortunately, developing these assays is often time-consuming and labor-intensive, as it depends on generating custom reagents or obtaining highly specific commercial ones. This dependence creates a bottleneck that hinders the rapid progression of drug discovery.

During the discovery phase of these biologics, pharmacokinetic (PK) and pharmacodynamic (PK-PD) studies are crucial for optimizing delivery and target engagement to identify lead candidates.

Off-the-Shelf Immunoassays that Save Time
Our IA team has developed and validated a “fit-for-purpose generic” ligand binding immunoassay for early pharmacokinetic (PK) assessment of human IgG-based biologics. This assay leverages reagents with specificities for the fragment crystallizable (Fc) region of human IgG antibodies to selectively capture and detect them across various species (see Figure 1). It can be employed as either an enzyme-linked immunosorbent assay (ELISA) or a high-sensitivity electrochemiluminescence (ECL) assay using the Meso Scale Discovery (MSD) platform.
In addition to measuring human IgG, this assay is versatile enough to quantify a range of fusion proteins containing at least one human IgG Fc domain, including bi-specific and tri-specific antibodies, antibody fusion proteins, complex multi-domain fusion proteins, and antibody-drug or nucleotide conjugates. The key benefits of this assay include its “off-the-shelf” availability, which eliminates the need for traditional method development and enables quick setup and rapid sample analysis. It is suitable for analyzing samples from mouse, rat, and non-human primate models.

Robust LLOQ Assay
This assay can be used to test various test articles, such as monoclonal, bi-specific, tri specific antibodies, antibody infusion proteins, multi domain fusion proteins and antibody drug/nucleotide conjugates. It has shown robust LLOQ, 50ng/mL by Plate Reader and 5ng/mL by MSD with as little as 5µL of sample volume.
(Figure 1) Specific target for human IgG based test substrates with at least a Fc domain Poly and mono clonal antibodies for “capture” and “detection”.
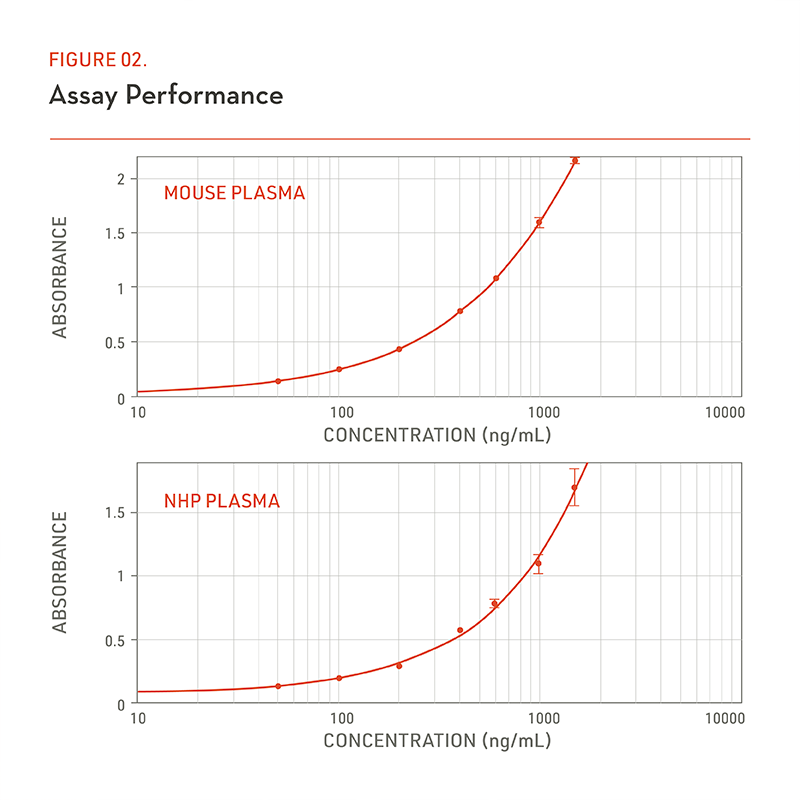
(Figure 2) Representative standard curves of generic human IgG assay in (a) mouse and (b) NHP plasma, respectively.

(Figure 3) Pharmacokinetic (PK) profile of human IgG administered intravenously (IV and SC) in mouse and rat.
Get Expert Opinions & The Latest Trends
Creating personalized paths that get therapies to marketing on time and on budget means staying ahead of the curve. Get thought provoking articles, expert insights and the latest news from our senior scientists.
